Judge Debunks Zantac Cancer Connection
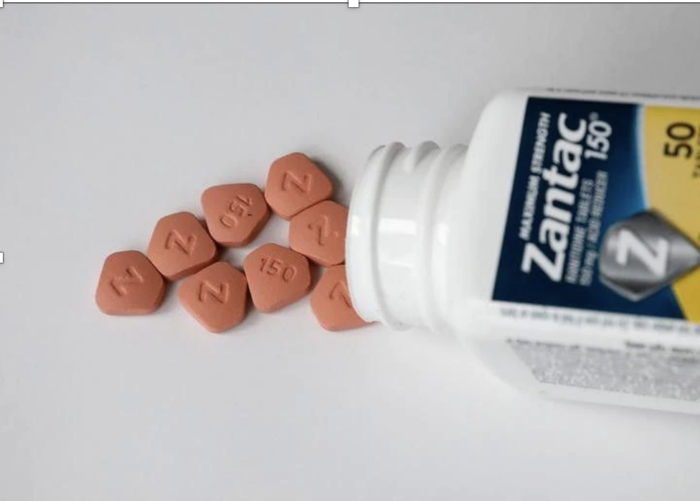
In a move that disappointed personal injury lawyers, US District Judge Robin Rosenberg dismissed 50,000 lawsuits that claimed Zantac caused cancer. Judge Rosenberg said the plaintiffs’ scientists “systemically utilized unreliable methodologies” to try and prove that ranitidine was carcinogenic. Plaintiffs’ attorneys had claimed that Zantac contained high levels of NDMA, a potentially carcinogenic nitrosamine.
In April 2020, the FDA requested removal of Zantac and all ranitidine products from the market because of NDMA concerns. You may recall that valsartan and other ARB’s were temporarily removed due to NDMA contamination, but none were permanently removed. NDMA can also be found in many processed foods and beverages such as whiskey, beer, cured meats, bacon, and cheeses.
I didn’t pay much attention the FDA announcement at the time—we had more pressing issues to deal with in the spring of 2020, but I was surprised when I learned that Zantac was permanently taken off the market.
So, why was Zantac—and generic ranitidine—removed so quickly? The decision was based on a “citizen petition” from Valisure testing lab that they found 3,000,000 ng of NDMA in pills—well above the accepted limit of 96ng. Even though the FDA tested pills and didn’t observe unacceptable levels of NDMA in many of the samples that they tested, the FDA immediately removed all ranitidine products.
A curious feature of Valisure’s citizen petition was that it released the same day numerous lawsuits were filed against drug manufacturers. This timing suggests coordination—or even collusion–between Valisure lab and personal injury lawyers.
Judge Robin Rosenberg did what the FDA failed to do. She thoroughly investigated the claims. As reported by the Wall Street Journal:
But as Judge Robin Rosenberg notes in her 341-page ruling, the FDA daily limit is “conservative”—equivalent to a meal of grilled meat. “If one were to consume 96 ng of NDMA every day, for 70 years in succession, the risk of cancer would be 1 in 100,000, or .001%,” and “even the highest-tested pill [by the FDA] showed NDMA at a tiny fraction of the level reported by Valisure.”
This important context was left out of lawsuits and press reports. Ditto that the FDA found Valisure’s lab equipment created NDMA. It gets worse, as Judge Rosenberg details. Valisure heated the ranitidine to 266 degrees Fahrenheit—well above the roughly 98 degree temperature found in the human body—to achieve its test result of 3,000,000 ng.
When Valisure tested ranitidine at 98 degrees, it found no NDMA. The extremely high temperature may have caused ranitidine to degrade into NDMA. Valisure also tested ranitidine’s reaction with salt in an artificial stomach, which resulted in NDMA levels exceeding 300,000 ng. But the enormous levels of salt in the test might alone have been enough to kill someone.
When Valisure tested ranitidine with salt concentrations approximating what a human could safely ingest, it detected no NDMA. The plaintiffs also relied on a Stanford University study that reported to find NDMA levels in ranitidine exceeding 47,000 ng. That study was later retracted by its authors after the lab equipment was found to have created NDMA.
“There is no scientist outside this litigation who concluded ranitidine causes cancer, and the Plaintiffs’ scientists within this litigation systemically utilized unreliable methodologies with a lack of documentation on how experiments were conducted, a lack of substantiation for analytical leaps, a lack of statistically significant data, and a lack of internally consistent, objective, science-based standards for the evenhanded evaluation of data,” wrote Judge Rosenberg, who was appointed by Barack Obama.
Valisure started business as a ‘digital pharmacy,’ but has now shifted over checking medications for contaminants. They present themselves as social do-gooders (like Sam Bankman-Fried did), but a review of their website shows that they were funded by venture capital and their main business is “Independent Product Certification.” The way I see it, the more products they can cast doubt on, the more business they can generate.
Valisure’s shoddy methods raise the question of whether to trust their recent finding of benzene in sunscreens, which gained a lot of publicity for them. I haven’t checked, but I suspect Valisure is developing a new revenue stream certifying sunscreens as benzene free.
Now, can we have our Zantac back?









I wish they’d put it back on the market. I found it to be the most effective of the H2 blockers.
Also, what is there to be said about the lawsuits regarding Acetaminophen use during pregnancy! I would not be surprised if lawyers started looking for people who drank water and developed cancer!
I found it ironic that one political party called itself the party of science. Yet that party receives 99% of the Plaintiff attorneys donations. Trials are almost always junk science. Many other countries the judge appoints the experts.
We legalize Marijuana smoking with carcinogens but sue Monsanto for billions because they can’t prove a negative, that Roundup never causes cancer, and thus anyone who used it gets cancer claims it was the cause, not their smoking, not their other genetics. At best it might cause one in a million of the cancers it has to pay out for.
I find it ironic in a country that the legal system costs 10 times as much per person as the second closest country that are health care only cost twice as much. That in itself is probably a miracle